Abstract
Acute myeloid leukemia (AML) is the most common type of hematological neoplasms in adults. TSC22 Domain Family Member 3 (TSC22D3) is one of glucocorticoid-induced genes and has an important regulatory role in immunosuppressive and cell proliferation. However, its prognostic value and immune infiltration effect in acute myeloid leukemia are remain elusive. Here, we investigated the association of TSC22D3 with immune infiltrates and its prognostic value in AML patients.
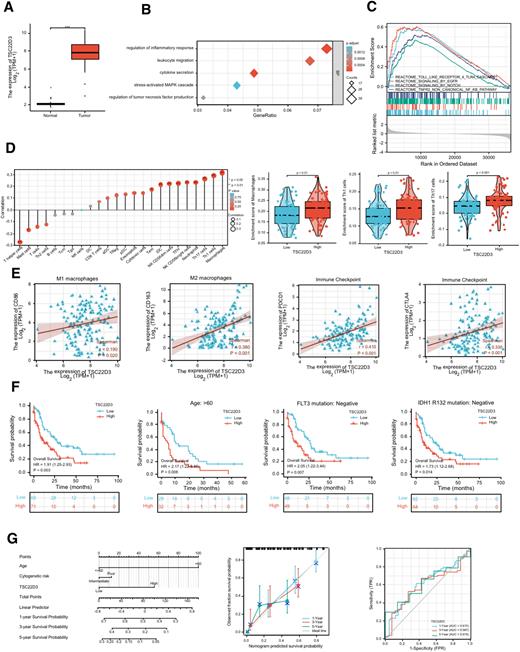
We first analyzed 173 patients from the TCGA and 70 healthy controls from GTEx databases and found that TSC22D3 was highly expressed in AML (Figure A)and closely associated with various biological processes and signaling pathways, including stress-activated MAPK cascade and proteoglycans in cancer and cytokine-cytokine receptor interaction (Figure B). In addition, GESA analysis demonstrated that TSC22D3 was closely corelated with several critical signaling pathways including Toll-like receptor 4 (TLR4) pathway, EGFR pathway, NOTCH pathway, TNFR2 non-canonical NF-κB pathway (Figure C), which further highlighted the important role of TSC22D3 in the pathogenesis of AML. Immunogen analysis found positive associations with various immune cells, especially macrophages (Figure D), and TSC22D3 was also significantly associated with PD-1 and CTLA-4 expression in AML (Figure E). Our results suggest that TSC22D3 is closely associated with immune injectors and promotes immune escape in AML patients in the tumor microenvironment. High level of TSC22D3 was related with poor prognosis of AML in age, BM blasts and several gene mutation (IDH1 R132, FLT3) subgroups (Figure F), indicated that TSC22D3 can be identified as a prognostic molecule in these clinical feature subgroups. Subsequently, a calibration nomogram based on the age and genetic risk level of the clinical patient was conducted. There was a favorable consistency between the actual observed and predicted values for 1-, 3-, 5-year OS based on the calibration chart (Figure G). Based on the complementary perspective for respective tumors, the constructed model provided a personalized score for individual patients.
Taken together, this study firstly reported that the high expression of TSC22D3 was significantly associated with the poor survival rate and immune infiltration in AML patients, which may promote the tumorigenesis of AML through enhancing the abnormal inflammation and immune escape. In particular, the immunomodulatory effects of TSC22D3 may play a crucial role in predicting the prognosis and have the potential to become a new biomarker of AML. Thus, this study identified that TSC22D3 may be useful as an immunomodulatory agent for AML clinical management and provided a new insight for further investigation on the clinicopathological significance and molecular pathogenesis of AML.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal